Our research program encompasses many areas of organic chemistry including synthetic methods development, total synthesis of natural products and medicinal chemistry. Projects in the group provide excellent training in organic synthesis and provide graduate students the opportunity to develop their creativity and critical thinking skills. In many cases, research in the group is aided significantly by computer modeling studies including DFT methods to predict reactivity/selectivity in methods projects and small-molecule docking for medicinal chemistry. Examples of areas of current interest are below.
Organocatalysis
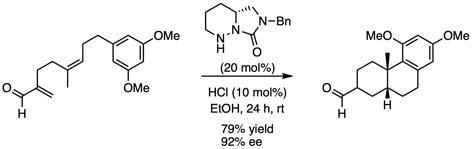
The use of small organic molecules as catalysts has many advantages over transition metal catalysts, including reduced costs, stability to both air and moisture, as well as decreased environmental and toxicological impacts. Our group is developing cyclic hydrazide catalysts which have the unique ability to catalyze a range of reactions with alpha-branched alpha,beta-unsaturated aldehydes. This has led to the first examples of iminium catalysis of the Cope rearrangement and polyene cyclizations. Current research is focussing on development of additional methodologies, including tandem oxy-Cope/Michael reactions.
Total Synthesis of Natural Products
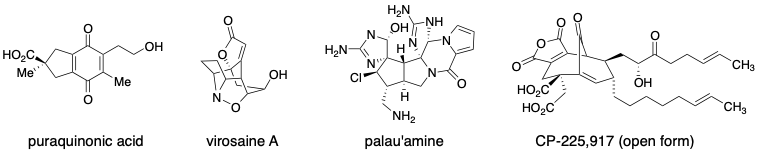
The total synthesis of natural products is a highly challenging endeavour which allows one to develop novel strategies, develop new chemistry and test developed methodology. We take on targets that either have unique structural features and/or possess functional arrays which allow us to examine novel methods developed within the group. A few targets of interest are shown below.
Multi-Action Drugs
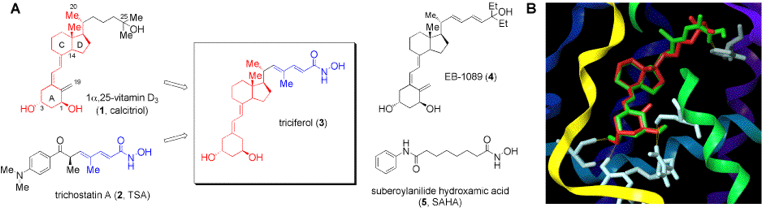
Traditionally, medicinal chemistry has focused on developing small-molecules which are selective for a single biological target and thus will be useful for treating a disease with a minimum of side-effects. In recent years, however, many clinical therapies have used combinations of drugs to treat a disease by focusing on several complimentary disease pathways. The best known example of this is using combinations of protease inhibitors and reverse transcriptase inhibitors to treat HIV. In a collaborative effort with groups in Physiology (McGill) and Biochemistry (University of Montreal), we are attempting to develop multi-action drugs which simultaneously target two synergistic biochemical pathways. This presents a significant challenge as we attempt to target two separate receptors and/or enzymes which have very different natural substrates and functions. However, through careful design, we have been able to develop small-molecules which can simultaneously target a nuclear receptor and a transcription regulator and are examining their effects on cellular models of cancer and autoimmune disorders.
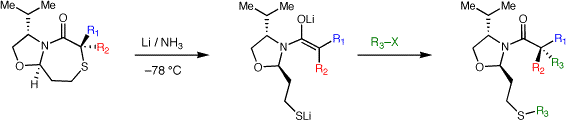
Chiral auxiliaries for stereoselective quaternary carbon formation
The formation of quaternary carbon stereocenters is a significant challenge in organic synthesis. When attempting to form quaternary carbon stereocenters through enolate alkylation reactions, a key problem to be addressed is enolate stereochemistry (E vs. Z isomers).While this is easily addressed in cyclic systems, E/Z enolate stereocontrol in acyclic enolates is very difficult. We have developed a method based on stereoselective reduction of alpha,alphas-disubstituted bicyclic thioglycolate lactams. Enolate stereochemistry is influenced by the conformation and stereochemistry of the bicyclic lactams. Our first system, developed as a proof-of-concept, was based on proline and has been improved upon in a second generation auxiliary which is formed readily from valinol, methyl thioglycolate and an acrolein equivalent. The auxiliary is currently available for purchase through Aldrich. The enolates from this second generation auxiliary may be alkylated to form simple quaternary carbon containing substrates and we are currently examining their use in aldol and Mannich reactions. This method has been successfully applied towards a highly efficient enantioselective synthesis of puraquinonic acid (see above).